Author's details
- Dr. Olohitai Gladys Ohiomoba,
- FWACS.
- FMC Ebute Metta, Lagos.
Reviewer's details
- Dr. Odofin James. T
- FWACS
- FMC Ebute Metta, Lagos.
- Date Uploaded: 2025-10-08
- Date Updated: 2025-12-18
Postpartum Haemorrhage: A Comprehensive Overview
Key Messages
- Postpartum haemorrhage (PPH) is the leading cause of maternal morbidity and mortality worldwide, with uterine atony responsible for about 70% of cases.
- Early recognition, rapid assessment, and prompt intervention, including resuscitation and identification of the underlying cause—are critical for effective management.
- The “four T’s” (Tone, Trauma, Tissue, Thrombin) summarize the main causes of PPH, and management should be tailored accordingly.
- Prevention relies on active management of the third stage of labour, skilled birth attendants, and access to appropriate medications and training.
Traditionally, postpartum haemorrhage (PPH) is defined as blood loss exceeding 500 mL following a vaginal delivery and more than 1,000 mL after an abdominal delivery. Clinically, however, any amount of blood loss with the potential to cause haemodynamic instability after delivery qualifies as PPH. The Royal College of Obstetricians and Gynaecologists (RCOG) classify PPH by the amount of blood lost, minor (500-1,000 mL) and major (>1,000 mL). In 2017, the American College of Obstetricians and Gynaecologists (ACOG) updated the definition to blood loss of greater than or equal to 1,000 mL, or blood loss accompanied by signs or symptoms of hypovolaemia within 24 hours after birth, regardless of delivery mode. The lack of a consistent definition has limited the ability to compare prevalence rates across studies, since estimated blood loss is often unreliable. Therefore, greater emphasis should be placed on evaluating the overall clinical status of the patient. Recent guidelines have begun to include the shock index and obstetric early warning systems for assessing bleeding and enabling early intervention.
PPH remains the leading cause of maternal mortality worldwide. According to the World Health Organization (WHO), PPH complicates approximately 2% of deliveries, leading to about 8% of maternal deaths in developing countries and up to 20% in developed countries. As one of the main direct causes of maternal mortality globally, reducing PPH is a central focus of Sustainable Development Goal 3 (SDG 3), which aims to reduce the global maternal mortality ratio to less than 70 per 100,000 live births by 2030. Achieving this target requires accelerating the annual reduction of maternal deaths, a challenging but vital task. PPH is generally categorised into two groups: Primary PPH, which occurs within the first 24 hours after delivery, and Secondary PPH, which occurs from 24 hours up to six weeks post-delivery. Most of the morbidity and mortality is associated with primary PPH.
There are several identifiable risk factors for PPH, although some instances occur unexpectedly. The four most common causes can be easily remembered as the "four T’s":
- Tone: Uterine atony
- Trauma: Genital tract trauma
- Tissue: Retained products of conception
- Thrombin: Coagulopathy
Uterine atony is responsible for roughly 70% of PPH cases. A wide range of risk factors are linked to PPH, including advanced maternal age, grand multiparity, previous PPH, history of antepartum haemorrhage in the current pregnancy, multiple pregnancy, polyhydramnios, macrosomia, hypertensive disorders, maternal anaemia, induction of labour, instrumental vaginal delivery, poor management of the third stage of labour, and uterine rupture. Risk factors for secondary PPH include endometritis, retained placental tissue, uterine fibroids, sub-involution, pseudo-aneurysms, and, rarely, arteriovenous malformations.
Pathophysiology
During pregnancy, uterine blood flow increases markedly, from around 100 mL/min in the non-pregnant state to about 700 mL/min. The primary protective mechanism for immediate haemostasis after delivery is myometrial contraction, which occludes uterine blood vessels—often referred to as the "living ligatures" of the uterus. This process impedes blood flow from the vascular space to the uterine cavity. Therefore, any conditions or risk factors that disrupt these protective mechanisms can result in PPH.
Clinical Evaluation
Accurate clinical assessment begins with the identification of risk factors through rapid history-taking and examination. Quantifying the volume of blood loss and determining the underlying cause are essential for prompt diagnosis and effective intervention. Signs of significant blood loss include tachycardia, tachypnoea, low oxygen saturation, and hypotension. As blood loss progresses, patients may feel cold, experience fainting episodes, and show symptoms of shock, such as confusion, blurry vision, clammy skin, and weakness. Notably, pregnant women may lose more than 1,000 mL of blood before hypovolaemia becomes apparent. Clinical signs often appear after the loss of more than 25% of blood volume (approximately 1,500 mL).
Visual estimation of blood loss is commonly used by clinicians but is prone to underestimation. More objective methods include gravimetric measurement, direct blood collection with suction devices, weighing surgical sponges and drapes, using graduated under-buttock drapes, and assessing clinical parameters. Some guidelines now recommend incorporating the shock index to evaluate the degree of haemorrhage.
A thorough examination should be conducted to identify the source of bleeding, including rapid assessment of uterine tone and inspection of the entire lower genital tract—vaginal walls, cervix, and labia—for lacerations or haematomas. The placenta should be checked for completeness.
Laboratory investigations play a role in assessing ongoing blood loss and guiding treatment decisions. Urgent serial packed cell volume (PCV), blood grouping, and cross-matching for possible transfusion should be initiated promptly. PPH laboratory panels typically include a complete blood count with platelet count, partial thromboplastin time, plasma thromboplastin, fibrinogen, and a comprehensive metabolic panel, alongside routine prenatal laboratory studies performed on admission.
Management
The management of PPH centers on patient resuscitation to maintain haemodynamic stability and ensure continued perfusion of vital organs, while identifying and addressing the underlying cause. The following approach is recommended:
- Call for help early.
- Resuscitate using the A-B-C-D approach to stabilise the patient.
- Establish two large-bore peripheral intravenous catheters (14- or 16-gauge) and administer crystalloid and colloid IV fluids as needed.
- Initiate protocols early for the release of blood products and massive transfusion protocols.
Uterine Atony
As the most common cause of PPH, uterine atony should be promptly assessed and managed. Haemostasis following placental separation relies on effective myometrial contraction. A soft uterus after delivery is indicative of uterine atony. The management steps include:
- Start uterine massage.
- Empty the urinary bladder.
- Administer uterotonic agents such as oxytocin, ergot alkaloids, and prostaglandins.
Oxytocin stimulates rhythmic contractions of the upper myometrium, constricting spiral arteries and reducing uterine blood flow. It is the first-line treatment for PPH, with an onset of action within 1 to 6 minutes following intravenous administration. The recommended dose is 10 IU intramuscularly or 20 IU diluted in 1 L of saline infused at 250 mL per hour.
Ergot alkaloids (e.g., ergometrine, ergonovine, methylergonovine) are serotonergic and partial α-adrenergic receptor agonists, causing sustained uterine contractions. The usual dose is methylergonovine 200 μg IM or IV; however, these are contraindicated in patients with hypertension.
Prostaglandin analogues include:
- Carboprost: A 15-methyl prostaglandin F2-α analogue, carboprost stimulates uterine contractions. The recommended dose is 250 μg IM or intramyometrially every 15 to 90 minutes, up to a maximum of 8 doses. It is contraindicated in severe hepatic, renal, or cardiovascular disease, and may cause bronchospasm in patients with asthma.
- Misoprostol: A prostaglandin E1 analogue with a prolonged onset of action depending on the route (oral, sublingual, rectal, or buccal). It should be avoided in patients on anticoagulant therapy or with cardiovascular disease. Adverse effects include nausea, diarrhoea, and fever.
Tranexamic acid (TXA), while not uterotonic, inhibits fibrinolysis and is often used alongside uterotonics. The recommended dose is 1 g IV over 10 minutes within 3 hours of delivery after a PPH diagnosis. TXA acts within 5 minutes and is contraindicated in patients with a history of hypercoagulopathy.
Additional interventions for uterine atony include bimanual compression, uterine tamponade, compression sutures (such as Esike’s three brace sutures and B-Lynch sutures), and hysterectomy.
Trauma
Birth trauma, such as perineal lacerations and haematomas, can result in significant blood loss and must be managed promptly. Bleeding sites should be identified and sutured if direct pressure fails to stop the bleeding. Episiotomies increase blood loss and should be avoided unless tears are imminent; however, timely repair is essential to minimise blood loss. Haematomas may be present as pain, swelling, or changes in vital signs disproportionate to observable blood loss. Small haematomas can be monitored, but larger or enlarging haematomas require incision, evacuation of the clot, irrigation, and ligation of bleeding vessels.
Uterine inversion, a rare complication of the third stage of labour, occurs in 0.05% of deliveries. It presents as a bluish-grey mass protruding from the vagina and may be accompanied by vital sign changes disproportionate to blood loss (Vaso-vagal effect), with the placenta often still attached. Following resuscitation, every effort should be made to replace the uterus quickly without detaching the placenta, either at the bedside or surgically. The Johnson method involves relaxing the uterus, grasping the fundus, and pushing it through the pelvis into the abdomen. Once repositioned, uterotonic agents should be administered to ensure uterine tone and prevent recurrence.
Uterine rupture, which occurs in 0.6 to 0.7% of vaginal births after caesarean section in women with a low transverse or unknown uterine scar, is rare in an unscarred uterus. Before delivery, it may be presented as fetal heart rate abnormalities, vaginal bleeding, abdominal tenderness, maternal tachycardia, circulatory collapse, or increasing abdominal girth. Symptomatic rupture requires surgical repair or hysterectomy. In the postpartum period, surgical exploration is recommended if rupture is suspected.
Tissue
Retained placental tissue or membranes can impair uterine contraction, necessitating examination of the placenta after expulsion. This is followed by manual uterine evacuation of clots or retained tissue. Retained placenta (failure to deliver the placenta within 30 minutes of birth) occurs in fewer than 3% of vaginal deliveries. Management options include umbilical vein injection of 20 mL saline with 20 units of oxytocin to reduce the need for manual removal or proceeding directly to manual removal with appropriate analgesia. If it is not possible to separate the placenta from the uterine wall, invasive placenta should be considered. Placenta accreta adheres to, increta invades, and percreta penetrates the myometrium. Conservative management, such as leaving the placenta in place or administering weekly oral methotrexate until β-hCG levels reach zero, may be attempted, but patients must be monitored for infection and late PPH. Surgical management involves hysterectomy in cases of invasive placenta.
Coagulopathy
Coagulation disorders are a rare but serious cause of PPH, resulting in consumptive coagulopathy. When coagulopathies are identified before delivery, multidisciplinary care and advanced planning are essential to prevent PPH. Risk factors include preeclampsia, von Willebrand’s disease, thrombocytopenic purpura, and haemophilia. Disseminated intravascular coagulation (DIC) may occur with severe preeclampsia, amniotic fluid embolism, sepsis, placental abruption, or prolonged intrauterine foetal demise. Consider coagulopathy in patients with cocaine use, those unresponsive to standard PPH treatments, or those with oozing from puncture sites. Evaluation should include platelet count, prothrombin time, partial thromboplastin time, fibrinogen, and fibrin split products (D-dimer). Management focuses on treating the underlying cause, supporting intravascular volume, serial coagulation assessment, and replacing blood components. Obstetric transfusion protocols use packed red blood cells, fresh-frozen plasma, and platelets in defined ratios to correct deficits, maintaining haemoglobin above 7–8 g/dL, fibrinogen above 2 g/L, and platelet count between 50,000 and 75,000/μL. Recombinant factor VIIa or clot-promoting agents like tranexamic acid may be considered.
Prevention
Health workers at all levels require access to appropriate medication and training in PPH prevention and management. Efforts should focus on reducing PPH through cost-effective, resource-appropriate interventions and minimising the need for expensive, life-saving surgical procedures. Routine use of active management of the third stage of labour by all attendants, regardless of practice location, is recommended.
Birth attendants must be skilled in providing safe, physiological management to prevent PPH in the absence of uterotonic drugs. Other preventive strategies include identifying and correcting anaemia before delivery, respecting the mother’s beliefs regarding blood transfusion, and avoiding routine episiotomy. Re-examining the patient’s vital signs and vaginal bleeding before leaving the delivery area may help detect ongoing slow bleeding. The most effective preventive measure is active management of the third stage of labour.
Conclusion
Postpartum haemorrhage continues to be the leading cause of maternal morbidity and mortality worldwide. Despite various collaborative efforts, there remains a lack of implementation and adherence to PPH management recommendations during obstetric emergencies. Delays are partly due to insufficient information from current evidence and the lack of unified guidelines for diagnosis and management. To address this, the FIGO Safe Motherhood and Newborn Health Committee, in collaboration with global experts, has produced this updated review. The aim is to provide clear, practical tools for diagnosing and managing PPH, tailored to institutional, local, and regional resource availability, particularly in low- and middle-income countries.
A case of a 26-year-old primigravida at 39 weeks of gestation who had 8 uneventful antenatal visits, presented to the labour ward with complaints of labour pains. She was assessed to be in the active phase of labour and progressed satisfactorily to have a spontaneous vaginal delivery of a live female neonate with a good Apgar score and a birth weight of 3.5 kg, with the aid of a median episiotomy. She, however, sustained a second-degree left intravaginal laceration that was promptly repaired. She was transferred to the postnatal ward.
After 4 hours of post-delivery, she complained of progressive, excruciating pain and swelling around the perineum; she was transferred back to the labour ward. General examination revealed a young woman in painful distress, not pale, afebrile, and not dehydrated. Her pulse rate was 100 bpm, and her blood pressure was 130/70 mmHg. The uterus was full, with no undue tenderness, and the uterine size was 20 cm. Perineal examination revealed gross swelling around the left labia measuring 10 × 8 cm, with marked tenderness. There was moderate bleeding per vaginum. A diagnosis of primary postpartum haemorrhage secondary to genital tract laceration was made.
She was resuscitated; urgent PCV was 28%, and two units of blood were requested. She underwent emergency genital tract exploration in the theatre under spinal anaesthesia, with the following findings:
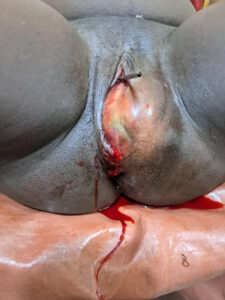
Figure: Normal external female genitalia with a huge swelling on the left perineum, measuring 10 × 8 cm, firm and differentially warm. The episiorrhaphy was intact and the cervix was normal. (Picture credit: Dr. Olohitai Gladys Ohiomoba)
An incision was made, approximately 600 mL of clot was evacuated, the cavity was irrigated, bleeding vessels were ligated, and the cavity was repaired with absorbable sutures. She received antibiotics, analgesics, oral ferrous sulphate, and routine sitz baths.
She was discharged and seen at the postnatal clinic with no new complaints.
- Dube R, Kar SS, Satapathy S, Bahutair SN, Younus HA, Abdulsalam KF. Risk Factors and Postpartum Hemorrhage among Women with Vaginal Delivery. Annals of African Medicine. 2025:10-4103.
- Moyo E, Dzinamarira T, Moyo P, Murewanhema G, Ross A. Magnitude and determinants of postpartum hemorrhage in sub-Saharan Africa: a systematic review and meta-analysis.
- Tochie JN, Fonkwo V, Njinkeu GK, Mbaya CH, Badjang TG. Challenges in the management of postpartum haemorrhage in sub-Saharan Africa. Acta Scientific Women’s Health. 2019;1(1):25-7.
- Escobar MF, Nassar AH, Theron G, Barnea ER, Nicholson W, Ramasauskaite D, Lloyd I, Chandraharan E, Miller S, Burke T, Ossanan G. FIGO recommendations on the management of postpartum hemorrhage 2022. International Journal of Gynecology & Obstetrics. 2022 Mar;157:3-50.
- Wormer KC, Jamil RT, Bryant SB. Postpartum hemorrhage. InStatPearls [Internet] 2024 Jul 19. StatPearls Publishing.
- Anderson JM, Etches D. Prevention and management of postpartum hemorrhage. American family physician. 2007 Mar 15;75(6):875-82.

More topics to explore
Author's details
Reviewer's details
Postpartum Haemorrhage: A Comprehensive Overview
- Background
- Symptoms
- Clinical findings
- Differential diagnosis
- Investigations
- Treatment
- Follow-up
- Prevention and control
- Further readings
Traditionally, postpartum haemorrhage (PPH) is defined as blood loss exceeding 500 mL following a vaginal delivery and more than 1,000 mL after an abdominal delivery. Clinically, however, any amount of blood loss with the potential to cause haemodynamic instability after delivery qualifies as PPH. The Royal College of Obstetricians and Gynaecologists (RCOG) classify PPH by the amount of blood lost, minor (500-1,000 mL) and major (>1,000 mL). In 2017, the American College of Obstetricians and Gynaecologists (ACOG) updated the definition to blood loss of greater than or equal to 1,000 mL, or blood loss accompanied by signs or symptoms of hypovolaemia within 24 hours after birth, regardless of delivery mode. The lack of a consistent definition has limited the ability to compare prevalence rates across studies, since estimated blood loss is often unreliable. Therefore, greater emphasis should be placed on evaluating the overall clinical status of the patient. Recent guidelines have begun to include the shock index and obstetric early warning systems for assessing bleeding and enabling early intervention.
- Dube R, Kar SS, Satapathy S, Bahutair SN, Younus HA, Abdulsalam KF. Risk Factors and Postpartum Hemorrhage among Women with Vaginal Delivery. Annals of African Medicine. 2025:10-4103.
- Moyo E, Dzinamarira T, Moyo P, Murewanhema G, Ross A. Magnitude and determinants of postpartum hemorrhage in sub-Saharan Africa: a systematic review and meta-analysis.
- Tochie JN, Fonkwo V, Njinkeu GK, Mbaya CH, Badjang TG. Challenges in the management of postpartum haemorrhage in sub-Saharan Africa. Acta Scientific Women’s Health. 2019;1(1):25-7.
- Escobar MF, Nassar AH, Theron G, Barnea ER, Nicholson W, Ramasauskaite D, Lloyd I, Chandraharan E, Miller S, Burke T, Ossanan G. FIGO recommendations on the management of postpartum hemorrhage 2022. International Journal of Gynecology & Obstetrics. 2022 Mar;157:3-50.
- Wormer KC, Jamil RT, Bryant SB. Postpartum hemorrhage. InStatPearls [Internet] 2024 Jul 19. StatPearls Publishing.
- Anderson JM, Etches D. Prevention and management of postpartum hemorrhage. American family physician. 2007 Mar 15;75(6):875-82.

Content
Author's details
Reviewer's details
Postpartum Haemorrhage: A Comprehensive Overview
Background
Traditionally, postpartum haemorrhage (PPH) is defined as blood loss exceeding 500 mL following a vaginal delivery and more than 1,000 mL after an abdominal delivery. Clinically, however, any amount of blood loss with the potential to cause haemodynamic instability after delivery qualifies as PPH. The Royal College of Obstetricians and Gynaecologists (RCOG) classify PPH by the amount of blood lost, minor (500-1,000 mL) and major (>1,000 mL). In 2017, the American College of Obstetricians and Gynaecologists (ACOG) updated the definition to blood loss of greater than or equal to 1,000 mL, or blood loss accompanied by signs or symptoms of hypovolaemia within 24 hours after birth, regardless of delivery mode. The lack of a consistent definition has limited the ability to compare prevalence rates across studies, since estimated blood loss is often unreliable. Therefore, greater emphasis should be placed on evaluating the overall clinical status of the patient. Recent guidelines have begun to include the shock index and obstetric early warning systems for assessing bleeding and enabling early intervention.
Further readings
- Dube R, Kar SS, Satapathy S, Bahutair SN, Younus HA, Abdulsalam KF. Risk Factors and Postpartum Hemorrhage among Women with Vaginal Delivery. Annals of African Medicine. 2025:10-4103.
- Moyo E, Dzinamarira T, Moyo P, Murewanhema G, Ross A. Magnitude and determinants of postpartum hemorrhage in sub-Saharan Africa: a systematic review and meta-analysis.
- Tochie JN, Fonkwo V, Njinkeu GK, Mbaya CH, Badjang TG. Challenges in the management of postpartum haemorrhage in sub-Saharan Africa. Acta Scientific Women’s Health. 2019;1(1):25-7.
- Escobar MF, Nassar AH, Theron G, Barnea ER, Nicholson W, Ramasauskaite D, Lloyd I, Chandraharan E, Miller S, Burke T, Ossanan G. FIGO recommendations on the management of postpartum hemorrhage 2022. International Journal of Gynecology & Obstetrics. 2022 Mar;157:3-50.
- Wormer KC, Jamil RT, Bryant SB. Postpartum hemorrhage. InStatPearls [Internet] 2024 Jul 19. StatPearls Publishing.
- Anderson JM, Etches D. Prevention and management of postpartum hemorrhage. American family physician. 2007 Mar 15;75(6):875-82.
Advertisement