Author's details
- Dr. Abdurrazzaq Alege
- MBBS, MBA (Healthcare Mgt), MPH, FMCPaed, IPNA fellow
Reviewer's details
- Dr Bello Afeez Oyesola
- MBBS, MPH, FMCPaed, IPNAfellow
- Date Uploaded: 2024-12-31
- Date Updated: 2025-12-20
Childhood Nephrotic Syndrome
Key Messages
- Nephrotic syndrome in children is characterized by massive proteinuria, hypoalbuminemia, generalized edema, and hyperlipidemia, most commonly due to idiopathic causes but can also be genetic, infectious, metabolic, or secondary to systemic diseases.
- Clinical presentation often includes periorbital and dependent edema, with complications such as infections, thrombosis, acute kidney injury, and side effects from medications like steroids and immunosuppressants.
- Diagnosis relies on history, physical examination, and laboratory investigations including urinalysis, serum proteins, and lipids.
- Management follows KDIGO 2024 guidelines, emphasizing steroids as first-line therapy, with definitions for remission, relapse, steroid sensitivity/resistance, and recommendations for steroid-sparing agents in frequent relapsers or steroid-dependent cases.
Nephrotic syndrome (NS) is a common childhood renal disorder resulting from abnormalities of the glomerular filtration barrier. It is characterised by excessive protein loss in the urine leading to low plasma level of protein and body swelling.
It is defined as a clinical entity characterised by the following:
Massive proteinuria: Urinary protein > 40mg/m2/hr or 1000 mg/m2/24hr or >300mg/dl or first morning urine protein/creatinine ratio ≥200 mg/mmol (2 mg/mg) corresponding to 3+ or 4+ by urine dipstick. Also, hypoalbuminaemia serum albumin <25 g/, generalized Oedema (Anasarca) and hyperlipidaemia: Serum cholesterol >200 mg/dl.
Proteinuria is due to increased filtration of macromolecules due circulating immune factors, mutations in podocyte or slit diaphragm proteins thereby creating a charge barrier leading to filtration of large anions such as albumin
Massive proteinuria causing albuminuria results in reduced intravascular oncotic pressure, the intravascular volume depletion leads to inhibition of atrial natriuretic peptide (ANP), activation of sympathetic nervous system (SNS) and renin-angiotensin-aldosterone system (RAAS) as well as release of antidiuretic hormone (ADH). All cause salt and water retention which worsens the oedema already due to shift of fluid to the extravascular compartment.
Hyperlipidaemia results from increased hepatic protein synthesis (including lipoproteins) due to hypoalbuminaemia. Increased urinary loss of lipoprotein lipase also results in decreased catabolism of lipids. The net effect is increased levels of serum lipids (cholesterol, triglycerides).
Nephrotic syndrome aetiology is mostly idiopathic. Factors such as genetic, environmental, haematologic, metabolic, systematic conditions and infections have all been implicated in the aetiology of the syndrome (Table 1).
Table 1: Aetiology and risk factors of nephrotic syndrome
| Idiopathic | Minimal change NS (MCNS), Focal segmental glomerulosclerosis (FSGS), membranous nephropathy, IgM nephropathy, membranous proliferative glomerulonephritis (MPGN), C1q nephropathy |
| Genetic | Finnish-type (NPHS1), familial FSGS (NPHS2), Denys-Drash syndrome (WT1), Pierson syndrome (LAMB2), isolated diffuse mesangial sclerosis (PLCE 1) and podocin gene (NPHS2) |
| Infections | Schistosomiasis, congenital syphilis, cytomegalovirus (CMV), Hepatitis B & C, HIV, Malaria, Filariasis and toxoplasmosis |
| Metabolic | Fabry’s disease, glutaric acidaemia, glycogen storage, mitochondrial cytopathies |
| Haematologic and Oncologic | Leukaemia, Lymphoma, sickle cell disease |
| Drugs | NSAIDs, gold, penicillamine, ACEIs, interferon, Mercury, Lithium |
| Systemic | Diabetes mellitus, lupus nephritis, amyloidosis, reflux nephropathy, Henoch Schoenlein purpura, |
| Others | Bee sting, food allergies, pregnancy |
Nephrotic syndrome can be classified based on aetiology as: (i) Congenital Nephrotic Syndrome (ii) Primary or Idiopathic Nephrotic Syndrome (iii) Secondary Nephrotic Syndrome. It can also be classified based on histological characteristics into: (i) Minimal change disease (ii) Focal segmental glomerulosclerosis (iii) Membranoproliferative glomerulonephritis (iv) Mesangial proliferative glomerulonephritis (v) Membranous nephropathy.
While congenital nephrotic syndrome manifests within the first 3 months of life, idiopathic nephrotic syndrome starts later and is most common between the ages of 2 and 7 years with a male preponderance. It is often heralded by a minor infection or allergic reactions including bee stings. It usually starts as a periorbital oedema usually worse in the morning. The oedema is also gravity dependent localized to the lower extremities in the standing position and to the dorsal part of the body in lying position. With time, the oedema becomes generalized, with the development of ascites, pleural effusions, and genital oedema.
Occurrence before 3 months of life and history of large placenta will suggest a congenital nephrotic syndrome. Other relevant history for the other forms include: bee sting, recent illness, drug and exposure to heavy metals, urine colour and volume as well as body rashes, and joint pains.
A general physical examination to identify the extent of oedema. Ascites is also a common abdominal finding. Blood pressure is usually normal with normal urine output. Extrarenal features, e.g., dysmorphic features or ambiguous genitalia or eye abnormalities (microcoria, aniridia), rash, arthritis. Other systemic examinations can be done to identify complications such as pleural effusion, pulmonary oedema from fluid accumulation.
Conditions that have a similar presentation to NS include acute or chronic glomerulonephritis, protein-losing enteropathy, oedematous severe acute malnutrition, congestive heart failure and hepatic failure.
H, Complications
Complications of nephrotic syndrome could be due to the disease or due to the complications following medications as indicated in Table 2 and 3 below.
Table 2: complications due to nephrotic syndrome
| Infectious | Spontaneous bacteria peritonitis usually due to organisms such as Streptococcus pneumoniae, Escherichia coli, Haemophilus influenzae, and Klebsiella spp. Viral infections and tuberculosis may be observed in patients receiving corticosteroids or immunosuppressive agents, cellulitis, urinary tract infection and disseminated varicella infection can also occur. |
| Cardiovascular | Hypertension, hyperlipidaemia, coronary artery disease |
| Respiratory | Pleural effusion and pulmonary embolism |
| Haematologic | Venous (more common) or arterial (less common) thrombosis (thrombo-embolic events due to urinary losses of Antithrombin III, Protein S and C. Also, haemoconcentration and immobilization) and anaemia (due to erythropoietin and transferrin loss) |
| Gastrointestinal | Intussusception |
| Renal | Acute kidney injury (usually pre-renal) and renal vein thrombosis |
| Endocrinologic | Reduced bone mineral density and hypothyroidism |
| Neurologic | Cerebral venous thrombosis |
Table 3: Complications of nephrotic syndrome due to medications
| Medications | Complications / side effects |
| Steroids | Growth impairment, avascular necrosis of femoral bone, posterior capsular cataract and |
| Alkylating agents | Haemorrhagic cystitis, dose related oligospermia, premature ovarian failure and increased risk of malignancy |
| Calcineurin agents | Gingival hyperplasia, hirsutism, hyperkalaemia, encephalopathy |
| MMF | Nausea, vomiting, diarrhoea, constipation, dose related leukopaenia and headache |
A good history makes it easy to diagnose nephrotic syndrome. Previous history of body swelling, description of urine and presence of risk factors assist in coining on the diagnosis. Routine investigations are urinalysis, urine protein: creatinine ratio, serum protein/albumin and fasting lipids. Complete blood count and serum electrolytes, urea and creatinine will serve as ancillary investigations. Figure 1
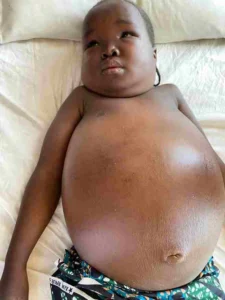 Figure 1: A girl with Nephrotic Syndrome (Picture credit Dr Aliu Rasaki Paediatric Nephrologist Federal Teaching Hospital Gombe)
Figure 1: A girl with Nephrotic Syndrome (Picture credit Dr Aliu Rasaki Paediatric Nephrologist Federal Teaching Hospital Gombe)
The kidney disease improving global outcome (KDIGO) 2024 treatment guideline is still relevant for NS management. However, key interventions before definitive treatment include:
Pneumococcal vaccine should be given if child will be admitted, also confirm status of exposure to varicella if to be treated in an open ward. Diet should entail increased protein intake, at least 120-140% protein of daily recommended allowance for age, with hypovolaemia give rapid infusion of plasma (20 ml/kg) or albumin 20% (1 g/kg) over 4 hours (Figure 2).
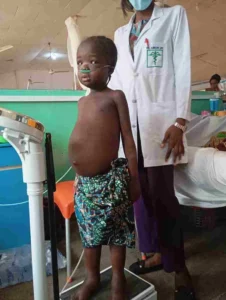 Figure 2: Same girl with nephrotic syndrome after a 4 day regime with albumin. (Picture credit Dr Aliu Rasaki Paediatric Nephrologist Federal Teaching Hospital Gombe)
Figure 2: Same girl with nephrotic syndrome after a 4 day regime with albumin. (Picture credit Dr Aliu Rasaki Paediatric Nephrologist Federal Teaching Hospital Gombe)
Salt restriction is necessary for massive oedema (To approximately 2 g of sodium per day). Fluid restriction should be implemented when serum Na is < 125meq/l. Diuretics (frusemide, hydrochlothiazide and spironolactone) are relevant when there is massive oedema and child is not hypovolaemic. Give empirical antibiotics once blood count suggests presence of infections.
Treatment of NS with steroid is the mainstay of treatment. To understand this in line with the KDIGO guideline there is a need to define the terms used as outlined below:
1. Remission: negative/ trace dipstick on ≥ 3 consecutive occasions.
2. Relapse: Urine dipstick ≥ 3+ (≥300 mg/dl) or UPCR ≥ 200 mg/mmol (≥ 2 mg/mg) on a spot urine sample on 3 consecutive days, with or without reappearance of oedema in a child who had previously achieved complete remission
3. Steroid-sensitive nephrotic syndrome (SSNS): Complete remission within 4 weeks of prednisone at standard dose (60 mg/m2 /day or 2 mg/kg/day, maximum 60 mg/day).
4. Steroid-resistant nephrotic syndrome (SRNS): Lack of complete remission within 4 weeks of treatment with prednisone at standard dose
5. Confirmation period: Time period between 4 and 6 weeks from prednisone initiation during which responses to further oral prednisone are ascertained in patients achieving only partial remission at 4 weeks. A patient not achieving complete remission by 6 weeks, although partial remission was achieved at 4 weeks, is defined as Steroid resistant nephrotic syndrome (SRNS).
6. SSNS late responder: A patient achieving complete remission during the confirmation period (i.e. between 4 and 6 weeks of prednisone therapy) for new onset NS.
7. Relapse: Urine dipstick ≥3+ (≥300 mg/dl) or UPCR≥200 mg/mmol (≥2 mg/mg) on a spot urine sample on 3 consecutive days, with or without reappearance of oedema in a child who had previously achieved complete remission.
8. Frequently relapsing nephrotic syndrome (FRNS): ≥ 2 relapses in the first 6-months following remission of the initial episode or ≥ 3 relapses in any 12 months.
9. Steroid-dependent nephrotic syndrome (SDNS): A patient with SSNS who experiences 2 consecutive relapses during recommended prednisone therapy for first presentation or relapse within 14 days of its discontinuation.
For SSNS it is recommended that oral prednisone be administered as a single daily dose starting at 60mg/m2 /d or 2mg/kg/d to a maximum 60 mg/d daily for 4–6 weeks, followed by alternate-day medication as a single daily dose starting at 40 mg/m2 or 1.5 mg/kg (maximum 40 mg on alternate days) for 2–5 months with tapering of the dose in the last month. All parents must be seen by the renal nurse / Paediatric Nephrologist for counselling details will include how, why and when to do urine dipstick testing, what a relapse is, what to do in the case of a relapse, understand the relapsing nature of nephrotic syndrome and learn about prednisolone, its dosing and side-effects. Following this treatment, 20% of children have no further episodes, 80% will relapse at least once and 50% will become frequent relapsers or steroid resistant.
For infrequent relapses of SSNS in children be treated with a single-daily dose of prednisone 60 mg/m2 or 2 mg/kg (maximum of 60 mg/d) until the child has been in complete remission for at least 3 days. After achieving complete remission, children be given prednisone as a single dose on alternate days (40mg/m2 per dose or 1.5mg/kg per dose: maximum 40mg on alternate days) for at least 4 weeks.
For steroid dependent or frequent relapsers, suitable steroid-sparing agents such as levamisole, alkylating agents or calcineurin inhibitors (CNIs). These medications are given along with low dose of prednisolone (20mg/m2 on alternate day for 3months). Levamisole is recommended to be given at a dose of 2.5 mg/kg on alternate days for at least 12 months. Alkylating agents (cyclophosphamide 2mg/kg over 2months with total maximum cumulative dose of 168mg/kg or chlorambucil 0.1-0.2mg/kg/day for 8weeks with cumulative dose of 11.2mg/kg). Calcineurin inhibitors (cyclosporin at 4–5mg/kg/d (starting dose) in two divided doses or tacrolimus at 0.1 mg/kg/d (starting dose) given in two divided doses) for at least 6 months. CNIs monitoring and alkylating agents for toxicity is very important.
For SRNS, it is highly recommended that corticosteroids treatments should be for a minimum of 8 weeks also administration of 3 doses of pulsatile doses of methyl prednisolone before finalizing on the diagnosis. A re-evaluation entailing a diagnostic kidney biopsy, evaluation of kidney function by GFR or eGFR and quantitation of urine protein excretion. Thereafter, administration of one of these three medications is advocated for SRNS:
1. Immunosuppressive (Calcineurin inhibitors, Mycophenolate mofetil, Rituximab)
2. Immune stimulatory: Levamisole
3. Non immunosuppressive: ACEi, ARBs and Vitamin E.
Renal biopsy should be done in steroid resistance children, other indications include children less than 1 year or more than 10 years of age, gross hematuria, sustained hypertension despite treatment, persistent renal insufficiency, low C3 component of complement, family history of renal failure and deafness as well as presence of extra-renal symptoms (Skin, Joints etc.).
Avoiding triggers or associations such as use of mercury, allergens (bee sting, house dust)
Control of infections: Reducing the burden of infections through vaccination, vector control (for malaria), and HIV prevention can help reduce secondary nephrotic syndrome.
Early diagnosis: Prompt and early recognition of signs and symptoms of nephrotic syndrome can improve outcomes.
Genetic counselling: For congenital nephrotic syndrome and families with a history of nephrotic syndrome, especially FSGS, genetic counselling may be useful in high-risk populations.
Childhood nephrotic syndrome in sub-Saharan Africa presents significant challenges due to limited healthcare resources and delayed access to medical care. Early diagnosis and proper management are crucial to prevent complications such as infections and kidney damage. Treatment typically includes corticosteroids, but recurrent relapses and steroid resistance can complicate the prognosis. Improved access to healthcare, early intervention, and better education for caregivers about the condition are essential to improving outcomes for children with nephrotic syndrome in the region.
A 4-year-old boy was brought to the emergency paediatric unit with complaints of 5 days history of generalised body swelling and painful micturition. The swelling started from the face, worse in the morning and subsequently involved the abdomen and the upper and lower limbs. There was a preceding fever with cough and catarrh; no body rash, passage of dark-coloured urine or reduction in urine output. Examination revealed a sick child who was mildly pale and had generalised oedema including the scrotum. Blood pressure was 80/40mmHg. A bedside urinalysis revealed protein 3+, leucocyte +, nitrite +, while other parameters were normal. The packed cell volume (PCV) was 29%. A rapid test for malaria was negative. A diagnosis of nephrotic syndrome with urinary tract infection was made. Blood samples for serum protein, albumin, lipids, electrolytes, urea and creatinine and a complete blood count and urine for microscopy and culture were sent to the laboratory. He was placed on intravenous furosemide 1mg/kg/dose 8 hourly, intravenous Amoxicillin. The mother was counselled to give the child a high protein diet and reduce salt intake. She was also informed that the child will commence high-dose steroid following laboratory results and after treatment of the urinary tract infection.
1. Aikhionbare H.A Owa J. A & Bugaje M. A, The Nephrotic Syndrome. In: JC Azubuike, Nkanginieme; Paediatric and Child Health in a Tropical Region. Educational Printing and Publishing (Publishers), Surulere, Lagos. 3rd Edition, 2016. ISBN: 978-81906735-01. Page 990-991.
2. Adedoyin, O. T., Buhari, M. O., Ibrahim, O. R., Oyedepo, O. O., Adesiyun, O. A., & Alege, A. (2024). Histopathologic Characteristics of Childhood Nephrotic Syndrome in a Tertiary Health Facility in Nigeria. West African Journal Of Medicine, 41(5), 493–498.
3. Esezobor, C., Ademola, A. D., Adetunji, A. E., Anigilaje E. A., Batte, A., Jiya-Bello F N., et al (2021). Management of idiopathic childhood nephrotic syndrome in sub-Saharan Africa: Ibadan consensus statement. Kidney International (2021) 99, 59–67.
4. Esezobor, C. I., Solarin, A. U., Gbadegesin, R (2020) Changing epidemiology of nephrotic syndrome in Nigerian children: A cross-sectional study. PLoS ONE 15(9): e0239300.
5. Noone, D., Wine, R., Vasilevska-ristovska, J., Banh, T., Knott, J., Noone, D., Gbadegesin, et al . (2021). Trends in the epidemiology of childhood nephrotic syndrome in Africa : A systematic review. Global Epidemiology 3 (2021) 10006.
6. Pais, P., & Avner E D., (2016). Nephrotic syndrome. In R.M. Kliegman, B.F. Stanton, J.W. St. Geme III, & N.F. Schor (Eds.), Nelson Textbook of Pediatrics (20th ed., pp.2521-2522). Elsevier.
7. Trautmann, A., Boyer, O., Hodson, E., Bagga, A., Gipson, D. S., Samuel, S. et al (2022). IPNA clinical practice recommendations for the diagnosis and management of children with steroid ‑ sensitive nephrotic syndrome. In Paediatric Nephrology. Springer.
8. KDIGO 2024 clinical practice guideline for the evaluation and management of chronic kidney disease. Supplement to kidney International 2024. Vol 105 (Suppl 45), 5117-5314.

More topics to explore
Author's details
Reviewer's details
Childhood Nephrotic Syndrome
- Background
- Symptoms
- Clinical findings
- Differential diagnosis
- Investigations
- Treatment
- Follow-up
- Prevention and control
- Further readings
Nephrotic syndrome (NS) is a common childhood renal disorder resulting from abnormalities of the glomerular filtration barrier. It is characterised by excessive protein loss in the urine leading to low plasma level of protein and body swelling.
It is defined as a clinical entity characterised by the following:
Massive proteinuria: Urinary protein > 40mg/m2/hr or 1000 mg/m2/24hr or >300mg/dl or first morning urine protein/creatinine ratio ≥200 mg/mmol (2 mg/mg) corresponding to 3+ or 4+ by urine dipstick. Also, hypoalbuminaemia serum albumin <25 g/, generalized Oedema (Anasarca) and hyperlipidaemia: Serum cholesterol >200 mg/dl.
Proteinuria is due to increased filtration of macromolecules due circulating immune factors, mutations in podocyte or slit diaphragm proteins thereby creating a charge barrier leading to filtration of large anions such as albumin
Massive proteinuria causing albuminuria results in reduced intravascular oncotic pressure, the intravascular volume depletion leads to inhibition of atrial natriuretic peptide (ANP), activation of sympathetic nervous system (SNS) and renin-angiotensin-aldosterone system (RAAS) as well as release of antidiuretic hormone (ADH). All cause salt and water retention which worsens the oedema already due to shift of fluid to the extravascular compartment.
Hyperlipidaemia results from increased hepatic protein synthesis (including lipoproteins) due to hypoalbuminaemia. Increased urinary loss of lipoprotein lipase also results in decreased catabolism of lipids. The net effect is increased levels of serum lipids (cholesterol, triglycerides).
1. Aikhionbare H.A Owa J. A & Bugaje M. A, The Nephrotic Syndrome. In: JC Azubuike, Nkanginieme; Paediatric and Child Health in a Tropical Region. Educational Printing and Publishing (Publishers), Surulere, Lagos. 3rd Edition, 2016. ISBN: 978-81906735-01. Page 990-991.
2. Adedoyin, O. T., Buhari, M. O., Ibrahim, O. R., Oyedepo, O. O., Adesiyun, O. A., & Alege, A. (2024). Histopathologic Characteristics of Childhood Nephrotic Syndrome in a Tertiary Health Facility in Nigeria. West African Journal Of Medicine, 41(5), 493–498.
3. Esezobor, C., Ademola, A. D., Adetunji, A. E., Anigilaje E. A., Batte, A., Jiya-Bello F N., et al (2021). Management of idiopathic childhood nephrotic syndrome in sub-Saharan Africa: Ibadan consensus statement. Kidney International (2021) 99, 59–67.
4. Esezobor, C. I., Solarin, A. U., Gbadegesin, R (2020) Changing epidemiology of nephrotic syndrome in Nigerian children: A cross-sectional study. PLoS ONE 15(9): e0239300.
5. Noone, D., Wine, R., Vasilevska-ristovska, J., Banh, T., Knott, J., Noone, D., Gbadegesin, et al . (2021). Trends in the epidemiology of childhood nephrotic syndrome in Africa : A systematic review. Global Epidemiology 3 (2021) 10006.
6. Pais, P., & Avner E D., (2016). Nephrotic syndrome. In R.M. Kliegman, B.F. Stanton, J.W. St. Geme III, & N.F. Schor (Eds.), Nelson Textbook of Pediatrics (20th ed., pp.2521-2522). Elsevier.
7. Trautmann, A., Boyer, O., Hodson, E., Bagga, A., Gipson, D. S., Samuel, S. et al (2022). IPNA clinical practice recommendations for the diagnosis and management of children with steroid ‑ sensitive nephrotic syndrome. In Paediatric Nephrology. Springer.
8. KDIGO 2024 clinical practice guideline for the evaluation and management of chronic kidney disease. Supplement to kidney International 2024. Vol 105 (Suppl 45), 5117-5314.

Content
Author's details
Reviewer's details
Childhood Nephrotic Syndrome
Background
Nephrotic syndrome (NS) is a common childhood renal disorder resulting from abnormalities of the glomerular filtration barrier. It is characterised by excessive protein loss in the urine leading to low plasma level of protein and body swelling.
It is defined as a clinical entity characterised by the following:
Massive proteinuria: Urinary protein > 40mg/m2/hr or 1000 mg/m2/24hr or >300mg/dl or first morning urine protein/creatinine ratio ≥200 mg/mmol (2 mg/mg) corresponding to 3+ or 4+ by urine dipstick. Also, hypoalbuminaemia serum albumin <25 g/, generalized Oedema (Anasarca) and hyperlipidaemia: Serum cholesterol >200 mg/dl.
Proteinuria is due to increased filtration of macromolecules due circulating immune factors, mutations in podocyte or slit diaphragm proteins thereby creating a charge barrier leading to filtration of large anions such as albumin
Massive proteinuria causing albuminuria results in reduced intravascular oncotic pressure, the intravascular volume depletion leads to inhibition of atrial natriuretic peptide (ANP), activation of sympathetic nervous system (SNS) and renin-angiotensin-aldosterone system (RAAS) as well as release of antidiuretic hormone (ADH). All cause salt and water retention which worsens the oedema already due to shift of fluid to the extravascular compartment.
Hyperlipidaemia results from increased hepatic protein synthesis (including lipoproteins) due to hypoalbuminaemia. Increased urinary loss of lipoprotein lipase also results in decreased catabolism of lipids. The net effect is increased levels of serum lipids (cholesterol, triglycerides).
Further readings
1. Aikhionbare H.A Owa J. A & Bugaje M. A, The Nephrotic Syndrome. In: JC Azubuike, Nkanginieme; Paediatric and Child Health in a Tropical Region. Educational Printing and Publishing (Publishers), Surulere, Lagos. 3rd Edition, 2016. ISBN: 978-81906735-01. Page 990-991.
2. Adedoyin, O. T., Buhari, M. O., Ibrahim, O. R., Oyedepo, O. O., Adesiyun, O. A., & Alege, A. (2024). Histopathologic Characteristics of Childhood Nephrotic Syndrome in a Tertiary Health Facility in Nigeria. West African Journal Of Medicine, 41(5), 493–498.
3. Esezobor, C., Ademola, A. D., Adetunji, A. E., Anigilaje E. A., Batte, A., Jiya-Bello F N., et al (2021). Management of idiopathic childhood nephrotic syndrome in sub-Saharan Africa: Ibadan consensus statement. Kidney International (2021) 99, 59–67.
4. Esezobor, C. I., Solarin, A. U., Gbadegesin, R (2020) Changing epidemiology of nephrotic syndrome in Nigerian children: A cross-sectional study. PLoS ONE 15(9): e0239300.
5. Noone, D., Wine, R., Vasilevska-ristovska, J., Banh, T., Knott, J., Noone, D., Gbadegesin, et al . (2021). Trends in the epidemiology of childhood nephrotic syndrome in Africa : A systematic review. Global Epidemiology 3 (2021) 10006.
6. Pais, P., & Avner E D., (2016). Nephrotic syndrome. In R.M. Kliegman, B.F. Stanton, J.W. St. Geme III, & N.F. Schor (Eds.), Nelson Textbook of Pediatrics (20th ed., pp.2521-2522). Elsevier.
7. Trautmann, A., Boyer, O., Hodson, E., Bagga, A., Gipson, D. S., Samuel, S. et al (2022). IPNA clinical practice recommendations for the diagnosis and management of children with steroid ‑ sensitive nephrotic syndrome. In Paediatric Nephrology. Springer.
8. KDIGO 2024 clinical practice guideline for the evaluation and management of chronic kidney disease. Supplement to kidney International 2024. Vol 105 (Suppl 45), 5117-5314.
Advertisement