Author's details
- 1. Dr. Nwala Gabriel Chuks 2. Dr. Garba Anita Ochanya 3. Dr. Uzuegbu Sonia Chiamaka
- 1. MBBCh (Calabar) FWACP (Paed), Cert, Paed Nutr (Boston) 2. MBBS. 3. MBChB.
- 1. Cert LMIH (Washington) Consultant Paediatrician, Limi Children's Hospital Abuja.. 2. Medical Officer, Limi Multispecialty Hospital, Abuja. 3. Medical Officer, Limi Multispecialty Hospital, Abuja, Nigeria.
Reviewer's details
- 1. Dr Ezurike Ezinwa Olekaibe 2. Dr Kephas Gurama Kabir
- MBBS, FWACP (Paed)
- 1. Consultant Paediatrician, Federal Medical Centre Umuahia, Abia State, Nigeria. 2. Federal Medical Centre, Jabi, Abuja.
- Date Uploaded: 2025-07-19
- Date Updated: 2025-12-20
Diabetic Ketoacidosis in a newly diagnosed Type 1 DM school boy: Challenges of Management in a resource limited setting
Key Messages
- Diabetic ketoacidosis (DKA) is a life-threatening emergency in children, often triggered by poor insulin compliance or infection, and requires meticulous fluid and electrolyte management, especially in resource-limited settings.
- Obesity does not exclude Type 1 diabetes mellitus (T1DM); overweight children can present with DKA, and differentiation from Type 2 diabetes may require autoantibody and C-peptide testing.
- Typical DKA symptoms include polyuria, polydipsia, weight loss, abdominal pain, vomiting, Kussmaul breathing, and altered mental status, with obese children possibly showing signs of insulin resistance.
- Management involves IV fluids, insulin infusion, aggressive potassium replacement, and careful monitoring for complications like cerebral edema, with long-term care focusing on insulin therapy, nutrition, and screening for comorbidities.
Diabetic Ketoacidosis (DKA) is a common life-threatening condition often seen in children with poor compliance with insulin or acute infective illness in resource limited facilities.
Limited or absent insulin production by the beta cells in patients’ pancreas, result in poor blood sugar control that leads to this life-threatening emergency.
Patients present with polyuria, polyphagia, polydipsia and varying degrees of altered sensorium. Blood sugar levels are usually more than 33mM/L and affected children are often in severe dehydration and altered consciousness.
Fluid resuscitation and electrolyte derangement can be either lifesaving, when meticulously done, or life threatening, if unscrupulously managed. All efforts should be applied to ensure the early identification of individuals with this condition and proper management.
Significance Of Obesity
While obesity often raises suspicion for Type 2 Diabetes, Type 1 diabetes mellitus (T1DM) can occur in overweight or obese children. A phenomenon increasingly seen with the obesity epidemic.
In children with DKA and obesity, careful differentiation is needed:
T1DM with insulinopenia may be presented similarly to T2DM in ketosis-prone patients.
Autoantibody testing (if available) and C-peptide levels (if accessible) can assist in classification later.
Clinical Presentation
Typical symptoms:
- Polyuria, polydipsia, weight loss
- Abdominal pain, vomiting
- Kussmaul breathing (deep, labored)
- Fruity-smelling breath
- Altered mental status in severe cases
Obese boys may also have signs of insulin resistance:
- Acanthosis nigricans
- Hypertension
- Dyslipidemia
Pathophysiology
- Insulin deficiency (absolute or relative) leads to:
- Hyperglycemia → osmotic diuresis → dehydration
- Lipolysis → free fatty acids → hepatic ketogenesis → acidosis
- In obese or overweight children:
- Insulin resistance + transient beta-cell dysfunction (due to glucotoxicity/lipotoxicity)
- DKA can be triggered by infection, stress, or missed diagnosis
Laboratory Features
- Hyperglycemia: Blood glucose >11 mmol/L (>200 mg/dL)
- Metabolic acidosis: pH <7.3, bicarbonate <15 mmol/L
- Ketosis: Blood ketones >3 mmol/L or moderate-severe ketonuria
Additional labs:
- Elevated anion gap
- Electrolyte imbalances (low Na+, variable K+)
- HbA1c (for chronic glycemic status)
- C-peptide and autoantibodies to distinguish T1DM vs T2DM (after recovery)
Management
Initial Stabilization
- Intravenous fluids: Correct dehydration (0.9% saline)
- Insulin infusion: 0.05–0.1 units/kg/hr (after fluids)
- Electrolytes: Monitor and replace K+ aggressively
- Glucose: Add dextrose to fluids when glucose <14–16 mmol/L
Monitor for:
- Cerebral oedema (especially in younger children)
- Hypoglycemia, hypokalemia
Long-term Management
- Insulin therapy
- Nutritional education, lifestyle modification
- Screen for comorbidities: NAFLD, dyslipidemia, hypertension
Conclusion
Management of DKA in resource limited settings can be challenging owing to some cultural, social, religious and emotional concerns of the care.
More public awareness campaigns and health education should be encouraged in developing countries like ours to improve the health seeking behavior, increase the uptake rate and reduce the adverse outcomes of DKA.
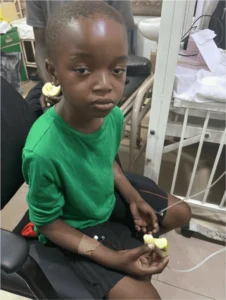
AN is a 7-year-old male that was rushed into the emergency room on account of abdominal pain, vomiting, fever and altered consciousness, noticed a day prior to presentation.
Abdominal pain was sudden in onset, sharp in character, colicky in nature, more over the epigastrium, non-radiating, not referred, but severe enough to affect child’s activity.
He had several episodes of non-bilious, non-projectile vomiting consisting of recently ingested meals.
At about the same time, he had a low grade on and off fever, worse at night, associated with chills and rigors, and relieved by paracetamol.
He also had a prior history of what parents called “frequent” urination, however no known history of change in the volume or colour of urine. There were
no associated lower urinary tract symptoms. His appetite was said to have increased but wasn’t gaining weight like his other two obese looking siblings despite his veracious appetite.
He was delivered healthy at term following an uncomplicated pregnancy and is the second child in a non-consanguineous marriage, No known family history of Type-I diabetes or any other chronic illness.
On examination, Child was conscious but drowsy, in mild respiratory distress with Kussmal breathing, tachypneic, with moderate palpebral conjunctival pallor, febrile (Axillary temp [37.7 0 C]), anicteric, severely dehydrated, dry buccal mucosa, sunken eyeballs, very slowly returning skin pinch, prolonged capillary refill time (>3seconds).
Vitals at arrival: T: 37.7 0 C, PR: 120bpm/ min small volume, rapid. Respiratory rate of 40cpm, Sp02: 98% in room air. Abdomen revealed marked Epigastric tenderness, no ascites or organomegally.
CNS: Conscious but drowsy, GCS: 13/1 5 BE:3/4, BV: 5/5, 8M: 5/6
Blood investigations available showed,
unrecordably high random blood glucose >
33.3mmol/dI (Acu-check),
Photospectometric values were 40mM/dI.
Full blood count showed TWBCC: 16.0x
1 O A9 (4-1 Ix 1 O A9) Absolute Neutrophil
(Polymorph) count: 12.12 x 1 0 A 9 (1.6-8.5 x
1 O A9). Packed cell volume: 33.8% (36- 54) Blood Film for Malaria parasites demonstrated asexual forms of P.falciparum (+)
Electrolyte Urea Creatinine: Na+: 1 25
(135-1 50mmoI/I), K+: 4.8mmol/I
(3.5–5.5mmoI/I), Chloride: 94mmoI/I (),
Bicarbonate: 7.1 mmol/l () Urea and serum
Creatinine level was within normal range Urinalysis; Ketone: +++, Glucose: ++, Protein: Trace, Blood: +.
Glycated Haemoglobin was 12% and C-peptide was 0.12.
Therefore, with the findings of polyphagia,? polyuria, hyperglycemia, leukocytosis, elevated HbA1C, low C-Peptide and urinalysis findings of glycosuria and ketonuria, a working diagnosis of DKA precipitated by sepsis? Focus and Malaria were made. Management immediately commenced, 2 IV accesses were secured for I.V fluid and insulin administration. He received about 2700mIs over 2-4hrs. The child commenced on soluble insulin after the first hour of fluid resuscitation 0.1 IU per kg/hr as a continuous infusion with hourly RBG monitoring. In addition, IV KCL was added to the IVF 1 OMEq/L after the second hour of fluid resuscitation and review of his serum potassium level (>3.5mM/L).
Injection Ceftriaxone +Tazobactam
(100mg/kg in 2 divided doses), I.V
Artesunate (Rekmal) 2.4mg/kg (64.8mg_)
@ 0, 1 2, 24hrs were also commencd. Child
was maintained on NPO until he was out of ketotic state evidenced by absence of ketonuria, regaining full consciousness and good sugar control. Vital signs and GCS were closely monitored.
Following the reduction of his RBG to < 14mM/dI, IVF N/S was changed to 5% D/S and insulin dosage reduced to 0.051U/Kg/ HR with sustenance of hourly RBS monitoring.
With his subsequent recovery of consciousness and clearance of ketonuria, he was encouraged to commence feeding with pre meal subcut soluble insulin 301U given 30minutes before his meals.
Random blood sugar was monitored at
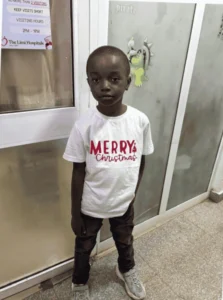
4hourIy interval, values fluctuated between 3.8 and 14mmoI/dI. This was due to his high caloric intake and informed the invitation of a dietician. Dietary counsel and modification were instituted by the Dietician with improvement in glycaemic control. He later commenced on a dual acting insulin (Mixtard) on the 4th day on admission and was later discharged on the 6th day following good glycaemic control and thorough counselling on the chronic nature of the condition, needed for compliance with medications, dietary plan and follow up visits.
He is doing well on medications and has been seen on a follow-up visit with good compliance on dietary plan and subcutaneous insulin. Blood sugar ranging between 3.8 and 8.0mM/dI.
This case emphasizes that DKA can occur even in children with a previously obese phenotype, challenging the common association of obesity with only Type 2 diabetes.
On admission
- American Diabetes Association. (2024). 14. Children and adolescents: Standards of Care in Diabetes—2024. Diabetes Care, 47(Suppl. 1), S254–S272. https://doi.org/10.2337/dc24-S014
- Craig, M. E., Jefferies, C., Dabelea, D., et al. (2014). Definition, epidemiology, and classification of diabetes in children and adolescents. Pediatric Diabetes, 15(Suppl. 20), 4–17. https://doi.org/10.1111/pedi.12186
- Dunger, D. B., Sperling, M. A., Acerini, C. L., et al. (2022). ISPAD Clinical Practice Consensus Guidelines 2022: Diabetic ketoacidosis and hyperglycemic hyperosmolar state. https://www.ispad.org/page/ISPADGuidelines
- Glaser, N. S., et al. (2001). Risk factors for cerebral edema in children with diabetic ketoacidosis. New England Journal of Medicine, 344(4), 264–269. https://doi.org/10.1056/NEJM200101253440404
- Wolfsdorf, J. I., Glaser, N., Agus, M., et al. (2020). ISPAD Clinical Practice Consensus Guidelines: Diabetic ketoacidosis in children and adolescents with diabetes. Pediatric Diabetes, 21(2), 145–157. https://doi.org/10.1111/pedi.13057
- American Diabetes Association. (2024). 14. Children and adolescents: Standards of Care in Diabetes—2024. Diabetes Care, 47(Suppl. 1), S254–S272. https://doi.org/10.2337/dc24-S014
- Dunger, D. B., Sperling, M. A., Acerini, C. L., et al. (2022). ISPAD Clinical Practice Consensus Guidelines: Diabetic ketoacidosis and hyperglycemic hyperosmolar state. Pediatric Diabetes, 23(S27), 133–153. https://doi.org/10.1111/pedi.13447
- Wolfsdorf, J. I., Glaser, N. S., & Agus, M. S. D., et al. (2020). Diabetic ketoacidosis and hyperglycemic hyperosmolar state. Pediatric Diabetes, 21(2), 145–157. https://doi.org/10.1111/pedi.13057
- Glaser, N. S., Barnett, P., McCaslin, I., et al. (2001). Risk factors for cerebral edema in children with diabetic ketoacidosis. New England Journal of Medicine, 344(4), 264–269. https://doi.org/10.1056/NEJM200101253440404
- Craig, M. E., Hattersley, A., Donaghue, K. C., et al. (2022). ISPAD Clinical Practice Consensus Guidelines: Definition and classification of diabetes in children and adolescents. Pediatric Diabetes, 23(S27), 7–20. https://doi.org/10.1111/pedi.13429
- Pulungan, A. B., Annisa, D., & Rahmadi, M. (2021). Challenges in managing diabetic ketoacidosis in low-resource settings. Journal of Pediatric Endocrinology and Metabolism, 34(1), 35–42. https://doi.org/10.1515/jpem-2020-0474
- Daneman, D. (2006). Type 1 diabetes. The Lancet, 367(9513), 847–858. https://doi.org/10.1016/S0140-6736(06)68341-4

More topics to explore
Author's details
Reviewer's details
Diabetic Ketoacidosis in a newly diagnosed Type 1 DM school boy: Challenges of Management in a resource limited setting
- Background
- Symptoms
- Clinical findings
- Differential diagnosis
- Investigations
- Treatment
- Follow-up
- Prevention and control
- Further readings
Diabetic Ketoacidosis (DKA) is a common life-threatening condition often seen in children with poor compliance with insulin or acute infective illness in resource limited facilities.
Limited or absent insulin production by the beta cells in patients’ pancreas, result in poor blood sugar control that leads to this life-threatening emergency.
Patients present with polyuria, polyphagia, polydipsia and varying degrees of altered sensorium. Blood sugar levels are usually more than 33mM/L and affected children are often in severe dehydration and altered consciousness.
Fluid resuscitation and electrolyte derangement can be either lifesaving, when meticulously done, or life threatening, if unscrupulously managed. All efforts should be applied to ensure the early identification of individuals with this condition and proper management.
- American Diabetes Association. (2024). 14. Children and adolescents: Standards of Care in Diabetes—2024. Diabetes Care, 47(Suppl. 1), S254–S272. https://doi.org/10.2337/dc24-S014
- Craig, M. E., Jefferies, C., Dabelea, D., et al. (2014). Definition, epidemiology, and classification of diabetes in children and adolescents. Pediatric Diabetes, 15(Suppl. 20), 4–17. https://doi.org/10.1111/pedi.12186
- Dunger, D. B., Sperling, M. A., Acerini, C. L., et al. (2022). ISPAD Clinical Practice Consensus Guidelines 2022: Diabetic ketoacidosis and hyperglycemic hyperosmolar state. https://www.ispad.org/page/ISPADGuidelines
- Glaser, N. S., et al. (2001). Risk factors for cerebral edema in children with diabetic ketoacidosis. New England Journal of Medicine, 344(4), 264–269. https://doi.org/10.1056/NEJM200101253440404
- Wolfsdorf, J. I., Glaser, N., Agus, M., et al. (2020). ISPAD Clinical Practice Consensus Guidelines: Diabetic ketoacidosis in children and adolescents with diabetes. Pediatric Diabetes, 21(2), 145–157. https://doi.org/10.1111/pedi.13057
- American Diabetes Association. (2024). 14. Children and adolescents: Standards of Care in Diabetes—2024. Diabetes Care, 47(Suppl. 1), S254–S272. https://doi.org/10.2337/dc24-S014
- Dunger, D. B., Sperling, M. A., Acerini, C. L., et al. (2022). ISPAD Clinical Practice Consensus Guidelines: Diabetic ketoacidosis and hyperglycemic hyperosmolar state. Pediatric Diabetes, 23(S27), 133–153. https://doi.org/10.1111/pedi.13447
- Wolfsdorf, J. I., Glaser, N. S., & Agus, M. S. D., et al. (2020). Diabetic ketoacidosis and hyperglycemic hyperosmolar state. Pediatric Diabetes, 21(2), 145–157. https://doi.org/10.1111/pedi.13057
- Glaser, N. S., Barnett, P., McCaslin, I., et al. (2001). Risk factors for cerebral edema in children with diabetic ketoacidosis. New England Journal of Medicine, 344(4), 264–269. https://doi.org/10.1056/NEJM200101253440404
- Craig, M. E., Hattersley, A., Donaghue, K. C., et al. (2022). ISPAD Clinical Practice Consensus Guidelines: Definition and classification of diabetes in children and adolescents. Pediatric Diabetes, 23(S27), 7–20. https://doi.org/10.1111/pedi.13429
- Pulungan, A. B., Annisa, D., & Rahmadi, M. (2021). Challenges in managing diabetic ketoacidosis in low-resource settings. Journal of Pediatric Endocrinology and Metabolism, 34(1), 35–42. https://doi.org/10.1515/jpem-2020-0474
- Daneman, D. (2006). Type 1 diabetes. The Lancet, 367(9513), 847–858. https://doi.org/10.1016/S0140-6736(06)68341-4

Content
Author's details
Reviewer's details
Diabetic Ketoacidosis in a newly diagnosed Type 1 DM school boy: Challenges of Management in a resource limited setting
Background
Diabetic Ketoacidosis (DKA) is a common life-threatening condition often seen in children with poor compliance with insulin or acute infective illness in resource limited facilities.
Limited or absent insulin production by the beta cells in patients’ pancreas, result in poor blood sugar control that leads to this life-threatening emergency.
Patients present with polyuria, polyphagia, polydipsia and varying degrees of altered sensorium. Blood sugar levels are usually more than 33mM/L and affected children are often in severe dehydration and altered consciousness.
Fluid resuscitation and electrolyte derangement can be either lifesaving, when meticulously done, or life threatening, if unscrupulously managed. All efforts should be applied to ensure the early identification of individuals with this condition and proper management.
Further readings
- American Diabetes Association. (2024). 14. Children and adolescents: Standards of Care in Diabetes—2024. Diabetes Care, 47(Suppl. 1), S254–S272. https://doi.org/10.2337/dc24-S014
- Craig, M. E., Jefferies, C., Dabelea, D., et al. (2014). Definition, epidemiology, and classification of diabetes in children and adolescents. Pediatric Diabetes, 15(Suppl. 20), 4–17. https://doi.org/10.1111/pedi.12186
- Dunger, D. B., Sperling, M. A., Acerini, C. L., et al. (2022). ISPAD Clinical Practice Consensus Guidelines 2022: Diabetic ketoacidosis and hyperglycemic hyperosmolar state. https://www.ispad.org/page/ISPADGuidelines
- Glaser, N. S., et al. (2001). Risk factors for cerebral edema in children with diabetic ketoacidosis. New England Journal of Medicine, 344(4), 264–269. https://doi.org/10.1056/NEJM200101253440404
- Wolfsdorf, J. I., Glaser, N., Agus, M., et al. (2020). ISPAD Clinical Practice Consensus Guidelines: Diabetic ketoacidosis in children and adolescents with diabetes. Pediatric Diabetes, 21(2), 145–157. https://doi.org/10.1111/pedi.13057
- American Diabetes Association. (2024). 14. Children and adolescents: Standards of Care in Diabetes—2024. Diabetes Care, 47(Suppl. 1), S254–S272. https://doi.org/10.2337/dc24-S014
- Dunger, D. B., Sperling, M. A., Acerini, C. L., et al. (2022). ISPAD Clinical Practice Consensus Guidelines: Diabetic ketoacidosis and hyperglycemic hyperosmolar state. Pediatric Diabetes, 23(S27), 133–153. https://doi.org/10.1111/pedi.13447
- Wolfsdorf, J. I., Glaser, N. S., & Agus, M. S. D., et al. (2020). Diabetic ketoacidosis and hyperglycemic hyperosmolar state. Pediatric Diabetes, 21(2), 145–157. https://doi.org/10.1111/pedi.13057
- Glaser, N. S., Barnett, P., McCaslin, I., et al. (2001). Risk factors for cerebral edema in children with diabetic ketoacidosis. New England Journal of Medicine, 344(4), 264–269. https://doi.org/10.1056/NEJM200101253440404
- Craig, M. E., Hattersley, A., Donaghue, K. C., et al. (2022). ISPAD Clinical Practice Consensus Guidelines: Definition and classification of diabetes in children and adolescents. Pediatric Diabetes, 23(S27), 7–20. https://doi.org/10.1111/pedi.13429
- Pulungan, A. B., Annisa, D., & Rahmadi, M. (2021). Challenges in managing diabetic ketoacidosis in low-resource settings. Journal of Pediatric Endocrinology and Metabolism, 34(1), 35–42. https://doi.org/10.1515/jpem-2020-0474
- Daneman, D. (2006). Type 1 diabetes. The Lancet, 367(9513), 847–858. https://doi.org/10.1016/S0140-6736(06)68341-4
Advertisement